POCUS and PE: A Deep Dive
- Dr. Istrail
- Mar 10, 2023
- 10 min read

The diagnosis of a pulmonary embolus (PE) with point-of-care ultrasound is a challenging one. As compared with pulmonary edema, pneumonia, or pleural effusion, the direct diagnosis of pulmonary embolism (PE) is more elusive. Since the primary pathology is located in the pulmonary arteries, in normal conditions they cannot be directly visualized well enough to diagnose PE reliably. However, with a honed clinical suspicion, appropriate use of a D-Dimer, and an excellent understanding of the downstream manifestations, a PE can be diagnosed using POCUS with accuracy approaching a gold-standard CT angiogram. This was shown in a 2014 study published in CHEST that used multiorgan ultrasound to diagnose PE with 90% sensitivity and 86% specificity. So how did they do it? To understand, let's take a step back and review the pathophysiology of a pulmonary embolus.
Pathophysiology
A pulmonary embolus is a blood clot that enters the pulmonary arterial circulation. It usually arises from the leg veins and travels up the IVC into the right heart where it is ejected into the pulmonary artery and downstream, to the segmental or subsegmental arteries.
This sudden occlusion of the pulmonary arteries by thrombus can lead to multiple downstream effects such as intra-alveolar hemorrhage or complete infarction with distal necrosis. These processes often involve the terminal air spaces and result in a subpleural, hypoechoic lesion that can often be seen with ultrasound (Figure 1 below).

Current tools available for diagnosis
There are three main non-sonographic diagnostic tools: Clinical suspicion/Wells' criteria, D-Dimer, and CT angiography.
Wells' criteria
The Wells' criteria is a set of objective and subjective clinical findings that are associated with a DVT or PE and should be assessed directly or indirectly in every patient presenting with chest pain or shortness of breath. They include leg swelling, history of DVT, recent surgery or leg immobilization, hemoptysis, or history of malignancy. It is a great starting point, yet even if every risk factor is positive, the incidence of PE was only 28-40%, and in the inpatient setting it performed even worse. In a study of 1100 inpatients, "the Wells score performed only slightly better than chance for discrimination of risk for DVT in hospitalized patients. It had a higher failure rate and a lower efficiency in the inpatient setting compared with that reported in the outpatient literature. Therefore, the Wells' score risk stratification is not sufficient to rule out DVT or influence management decisions in the inpatient setting." It is further weakened by the fact that the original Wells' criteria were validated against V/Q scans, which artificially inflates the diagnostic accuracy (since they are less sensitive studies) compared with the current CT angiography era.
D-Dimer
The D-dimer is a degradation product of a cross-linked fibrin blood clot. D-Dimer can be elevated for many other reasons besides clotting, so it is not specific to DVT or PE. However, it is incredibly sensitive, meaning that the d-dimer can be a superb test for ruling out DVT or PE if it is completely normal. The sensitivity of a d-dimer ranges from 95-99%, meaning that if it is negative, a DVT or PE can be essentially ruled out. Yet more often than not, the D-dimer is slightly or moderately elevated, leaving us in a diagnostic grey zone.
CT angiography
The gold standard to diagnose PE is CT angiography (CTA). They are easy to order, quick to complete, and highly accurate, making them an incredibly useful tool for diagnosing PE. CTAs have a sensitivity and specificity of over 90% and are able to detect small PEs in the distal, subsegmental arteries. Yet this ease of use is accompanied by multiple downsides that are rarely discussed.
Low positivity rate, radiation, contrast administration
While the CTA is the best method to diagnose a PE, in the real world it takes about 10 to 20 CT scans to be done to diagnose one PE. As the radiologists in one study concluded, "although a definitive acceptable PE positivity rate for CTA has not been established, we believe that the rate of 10% represents overuse of CTA as a screening rather than a diagnostic test." Each of these scans exposes the patient to about 10 mSv - 100 chest x-rays worth - of ionizing radiation, which with repeated exposures can be carcinogenic. We know that 50-100mSv of ionizing radiation exposure over a protracted period can increase cancer risk. Over a 4 year period in one study of 300 patients, the mean cumulative 4-year effective radiation dose was 31 mSv but ranged anywhere from 7 mSv from a single CT scan to 297 mSv from 12 CT scans during that period. This is especially concerning in children since their cells are more rapidly dividing and they have more decades left for cancer to develop. CTAs also require IV contrast administration which is generally accepted to cause kidney injury in people with acute or chronic kidney disease, and rarely causes anaphylaxis. This begs the question:
Are we overusing CTAs? Are we overdiagnosing PEs?
One JAMA review article says yes.

As seen in the above figure, a true increase in disease incidence would result in more nonfatal PEs diagnosed, corresponding to more fatal PEs. An ideal screening test would result in more early PE detection with less resulting mortality, suggesting you are appropriately catching early PEs and treating them to prevent death. In contrast, if overdiagnosis is occurring, more PEs are diagnosed with no change in mortality. As was seen in the United States, since the high-sensitivity CTs were introduced, the incidence has increased, which decreases the case fatality rate (increases the denominator), with minimal change in mortality.

As the researchers explained, "If the extra emboli diagnosed were clinically important and benefited from treatment, mortality (i.e., number of fatal pulmonary emboli/population at risk) would show a parallel decrease. This is exactly what happened in the 20 years prior to the introduction of CTPA: with improved prevention and treatment, PE mortality in the United States fell 50%, decreasing by 970 deaths per 100,000. By contrast, in the 8 years since CTPA was introduced, despite the large increase in new cases, mortality decreased by only another 0.4 deaths per 100,000. Mortality changed little because many of the extra emboli may not have needed treatment at all."
Very similar results were described in a NEJM study examining melanoma diagnosis trends. The incidence of melanoma is now 50 times as high as it was in 1975 (25 vs. 0.5 per 100,000 population). This rising detection and treatment of local melanoma has failed to reduce the incidence of invasive melanoma and has been accompanied by very steady mortality. This stone-cold steady mortality coupled with steeply rising incidence"should be viewed as pathognomonic for overdiagnosis.”

The evidence for PE overdiagnosis was supported by another review comparing single versus multi-detector CTAs (which are more sensitive) to diagnose PE. They found that the multi-detector CTAs were in fact more sensitive for diagnosing subsegmental PEs. Yet the 3‐month thromboembolic risks in patients with suspected PE and negative single or multi-detector CTA were the same, suggesting the additional sensitivity isn't missing clinically meaningful clots.
This was echoed by another retrospective study, and a 2017 review by hematologists looking at the evidence for and against treating symptomatic, subsegmental PEs, concluding that "symptomatic subsegmental PEs might be safely managed without the use of anticoagulation." This is also consistent with the 2016 CHEST guidelines, which recommend clinical surveillance over anticoagulation.
The reason this is important is that in order to understand how useful POCUS may be in diagnosing clinically significant PEs, we must understand what it is being compared to. As tests get better and more sensitive, we always run the risk of diagnosing clinically insignificant findings and inappropriately shuttling more and more healthy people into the medical treatment pipeline.
Lung ultrasound in the diagnosis of PE
There are vascular and pleural findings that can be seen with a PE, but the most characteristic and useful findings are parenchymal ones. They can be used to differentiate a consolidation resulting from a PE as compared to other pulmonary lesions like pneumonia (Figure 2 below).
These characteristics are as follows:
Most lesions resulting from a PE are hypoechoic, pleural-based, parenchymal alteration
Most are localized in an area of pleuritic chest pain and have a wedge shape more than 85% of the time, with well-demarcated margins
The lesions have a hypoechoic, homogenous sonomorphology
The majority are found in the right lower lobe -- can have multiple such lesions, most of which occur in the lower lobes (80% of the time) with a preference for the right lung.
As seen below in an example of a young patient with confirmed PE, her peripheral lesion had the following appearance:
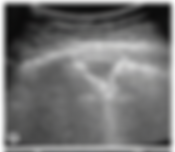
This shape is juxtaposed with that of pneumonia or a carcinoma which are more irregularly shaped and often non-homogenous sonomorphology. Below is another example of a triangular-appearing, pleural-based, hypoechoic lesion with clear margins and corresponding parenchymal and arterial findings on the CTA.

In a study of 69 patients, a lung ultrasound was performed looking for the above criteria and the diagnostic accuracy was compared to a spiral CT. 85% of the pulmonary lesions detected were triangular in shape. POCUS was able to diagnose PEs with 80% sensitivity and 92% specificity. This is compared to a spiral CT, which is less sensitive than the current gold standard of CT angiography for clots in the smaller, subsegmental arteries. As the authors explained, "although [spiral CT] scanning offers high sensitivity and specificity for central or segmental PEs, more peripheral thromboembolic lesions confined to the subsegmental level may be overlooked." With more modern CTA techniques, the sensitivity and specificity of diagnosing a PE are now over 90%.
This brings us back to the study at hand, published in CHEST: Accuracy of Point-of-Care Multiorgan Ultrasonography for the Diagnosis of Pulmonary Embolism.
They recruited patients with elevated Wells' scores or a positive D-dimer and performed a multiorgan POCUS exam: lung exam looking for subpleural infarcts, cardiac POCUS looking for right ventricular dilation, and a lower extremity venous ultrasound looking for DVT. The exam was considered positive if at least one of the following was seen:
Lung exam:
Anterior and posterior lung looking for pleural-based, well-demarcated echo-poor, triangular or rounded consolidations at least 0.5cm in size.
Heart exam / RV dilation diagnosed with at least one of the following:
Parasternal long-axis view: RV end-diastolic diameter > 30 mm
Apical 4-chamber or subcostal view: right/left ventricular end-diastolic diameter ratio of > 0.9
Leg vein doppler:
short axis visualization and lack of compression in the common femoral, superficial femoral, and popliteal veins
Out of the 357 patients included in the final results, PE was diagnosed by CTA in 110 of them. Some interesting findings:
Among the 175 patients with a Wells' score over 4, PE was present in less than half (72)
D-dimer was negative in 4% of patients with a PE
77 patients had at least one subpleural infarct, with a mean of 2.1 -- in 87% of these patients, a PE was confirmed with CTA. The infarcts were located mainly in the posterior lower lobes, with a small preference for the right lung:

62% of patients with RV enlargement had a PE
90% of patients with lower extremity DVT had a PE
99 out of the 133 patients with positive multiorgan exams had a PE
11 out of 224 patients with negative multiorgan ultrasound exams still had a PE
None of the patients with negative D-dimer and negative multiorgan ultrasound exam had a PE, which is why they propose including a multi-organ POCUS exam in a diagnostic algorithm to rule out PE with certainty:

Other POCUS Signs
Other cardiac signs associated with PE are notably specific but NOT sensitive for PE, therefore if you see it, PE is more likely, but it can't be used to rule one out.
McConnell's sign
In 1996, Dr. Michael McConnell and his colleagues published Regional Right Ventricular Dysfunction Detected by Echocardiography in Acute Pulmonary Embolism, describing a new right ventricular finding of "akinesia of the mid-free wall but normal motion at the apex" seen in patients with acute PE. They described it in a small cohort of patients with PE, then used it to evaluate 85 hospitalized patients with right ventricular dysfunction of any cause. It has now become known as the McConnell sign, and they reported a sensitivity of 77% and specificity of 94% for diagnosing acute PE. However, a retrospective blinded study from 2005 looked at the echos of patients who had massive PE, submassive PE, or right ventricular infarct and found that the McConnell sign was not nearly as accurate. It was present in 70% of patients with acute PE and 67% of patients with RV infarction and was only 70% sensitive and 33% specific for acute PE in this population. For more on this sign, here is a great lecture on it:
RVOT doppler evaluation
Since the right ventricle pumps blood directly into the pulmonary artery, acute or chronic changes in the afterload in the pulmonary artery can alter the blood velocity and flow that can be observed with doppler. In the parasternal short-axis view at the aortic valve level, if the pulse wave doppler gate is placed in the RV outflow tract (RVOT), insight into the pulmonary artery pressures will appear. One variable to look at is the acceleration time, which measures the slope of the doppler signal. Pulmonary artery pressure is linearly related to the pulmonary velocity acceleration time (PVAT):

Practically it can be very hard to measure the acceleration time since it is not always clear exactly what pixel to use for the beginning and the end measurement. Tiny variations can result in large differences in the calculation. A more practical method is to look at the shape of the right ventricular outflow doppler envelope. A study of 120 patients showed that shape is an excellent predictor of pulmonary pressure. As the signal gets more triangular, as the pressure increases:

They found that:
Pattern I: can be found in patients with PASP up to 47 mmHg with a 98% sensitivity and 100% specificity (AUC 0.999, P < 0.0001).
Pattern II: PASP between 48 and 68 mmHg with a 85% sensitivity and 96% specificity (AUC 0.960, P< 0.0001)
Pattern III: PASP between 69 and 94 mmHg with a 94% sensitivity and 100% specificity (AUC 0.995, P < 0.0001)
Pattern IV: PASP > 95 mmHg with a 100% sensitivity and 100% specificity (AUC1.0, P <0.0001)

Early systolic notching (Pattern III from above)
As seen in pattern III from the study above, the doppler signal assumes a more triangular shape and develops a notching pattern seen on the ascending portion:

Early systolic notching was observed in a retrospective study of patients with confirmed PE, Early systolic notching was seen in 92% of patients with massive PE (hemodynamic instability), or submassive PE (RV dysfunction but no hemodynamic instability), 2% with subsegmental PE, and not seen in patients without PE.

These retrospective findings were followed up with a prospective study from 2022 which found that early systolic notching was 34% sensitive and 97% specific for PE. The RVOT doppler was considered positive for early systolic notching if it "exhibited a sharp notch after a narrow spike within the first half of systole, followed by a second curvilinear (dome) Doppler signal" as seen below.

This prospective study of patients presenting with a pulmonary embolus found that:
Dyspnea was the most common complaint (48%) followed by back and chest pain
Early Systolic Notching was seen in 20% of patients, and 30 of the 33 had an acute pulmonary embolus
Patients with Early Systolic Notching were more likely to have a large PE and be categorized as intermediate or high risk
So in summary, POCUS adds many tools that - combined with a d-dimer can diagnose or rule out a pulmonary embolus in most patients. It is important to remember that even with a negative multiorgan POCUS exam, 11 of the 224 patients still had a pulmonary embolus. Like with everything POCUS, we should learn and understand the techniques and be very cognizant of its limitations.



